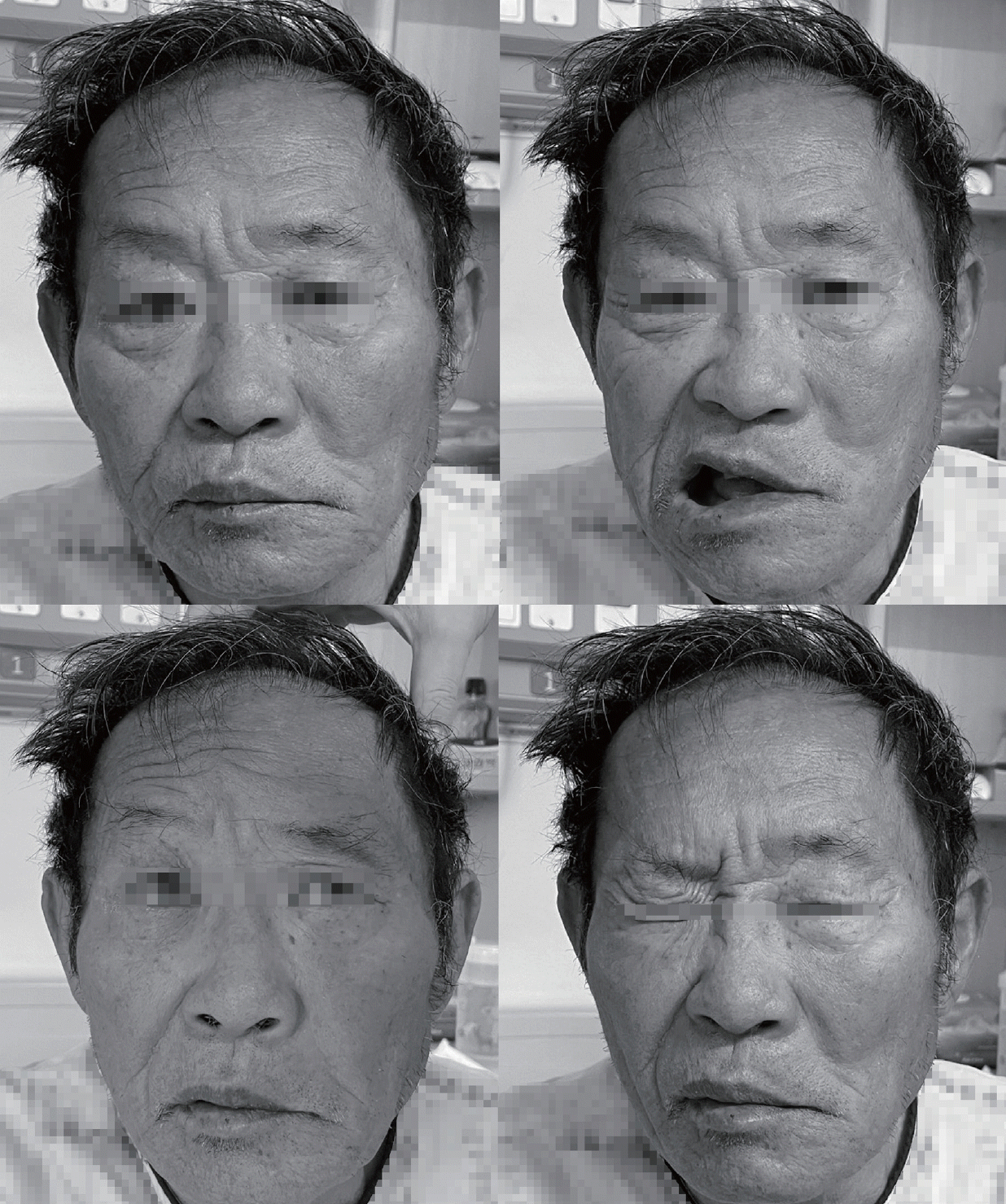전하소뇌동맥 영역의 급성 허혈성 뇌졸중 후 발생한 지연성 말초성 안면마비
Delayed Peripheral Facial Palsy after Acute Ischemic Stroke in the Territory of Anterior Inferior Cerebellar Artery
Article information
Trans Abstract
A 76-year-old male presented with dizziness and disequilibrium. Magnetic resonance imaging revealed an acute ischemic stroke in the left anterior inferior cerebellar artery (AICA) territory. Three days after admission the patient developed peripheral facial palsy with no radiological exacerbation of the infarction. He was managed with antiplatelet therapy and supportive care. Both the facial palsy and initial cerebellar symptoms resolved within 1 month. This case highlights delayed facial palsy as a rare presentation of AICA infarction.
The anterior inferior cerebellar artery (AICA) supplies the cerebellum, pons, and inner ear. Symptoms such as ataxia, vertigo, auditory disturbances, and peripheral facial palsy may manifest when these regions are affected by an acute ischemic stroke (AIS) [1-3]. However, the onset of these symptoms may be delayed due to variations in the susceptibility of different neural structures to ischemic injury. We present a case of delayed peripheral facial palsy in a patient with AIS in the AICA territory and discuss the potential lesion location for the development of peripheral facial palsy.
CASE REPORT
A 76-year-old male was admitted to our hospital due to the sudden onset of dizziness and disequilibrium 7 days previously. His neurological status had deteriorated, and he had been unable to walk independently for the past 5 days. The patient had a history of hypertension that had been managed with medication, and he had no known history of diabetes mellitus, cardiac disease, or previous stroke. In the initial neurological examination the patient was fully alert but had slight dysarthria. Extraocular movements were normal with the exception of gaze-evoked nystagmus being more severe on the left side. Sensations to light touch and pinprick were intact throughout the body, including the face. Despite the patient not complaining of any hearing disturbance, Weber and Rinne tests indicated sensorineural hearing loss on the left side. The muscle strength was normal but the patient displayed bilateral appendicular ataxia, which was more severe in the left limbs. His gait was unsteady and he was unable to walk in tandem. Diffusion-weighted imaging of the brain showed acute ischemic lesions in the left middle cerebellar peduncle and the left lateral pons corresponding to the territory of the AICA (Fig. 1-A). A focal chronic infarction was also observed in the right cerebellum. Magnetic resonance (MR) angiography revealed no abnormalities in the bilateral vertebral arteries or the basilar artery (BA), but there was a cut-off appearance of the left AICA where it originated from the BA (Fig. 1-C). Mild bilateral stenosis was also observed in the mid-M1 segments of the middle cerebral arteries. Hematological and cardiac investigations showed no abnormalities. Measuring the lipid profile revealed total cholesterol at 161 mg/dL, triglycerides at 201 mg/dL, high-density lipoprotein (HDL) cholesterol at 32 mg/dL, and low-density lipoprotein (LDL) cholesterol at 110 mg/dL.
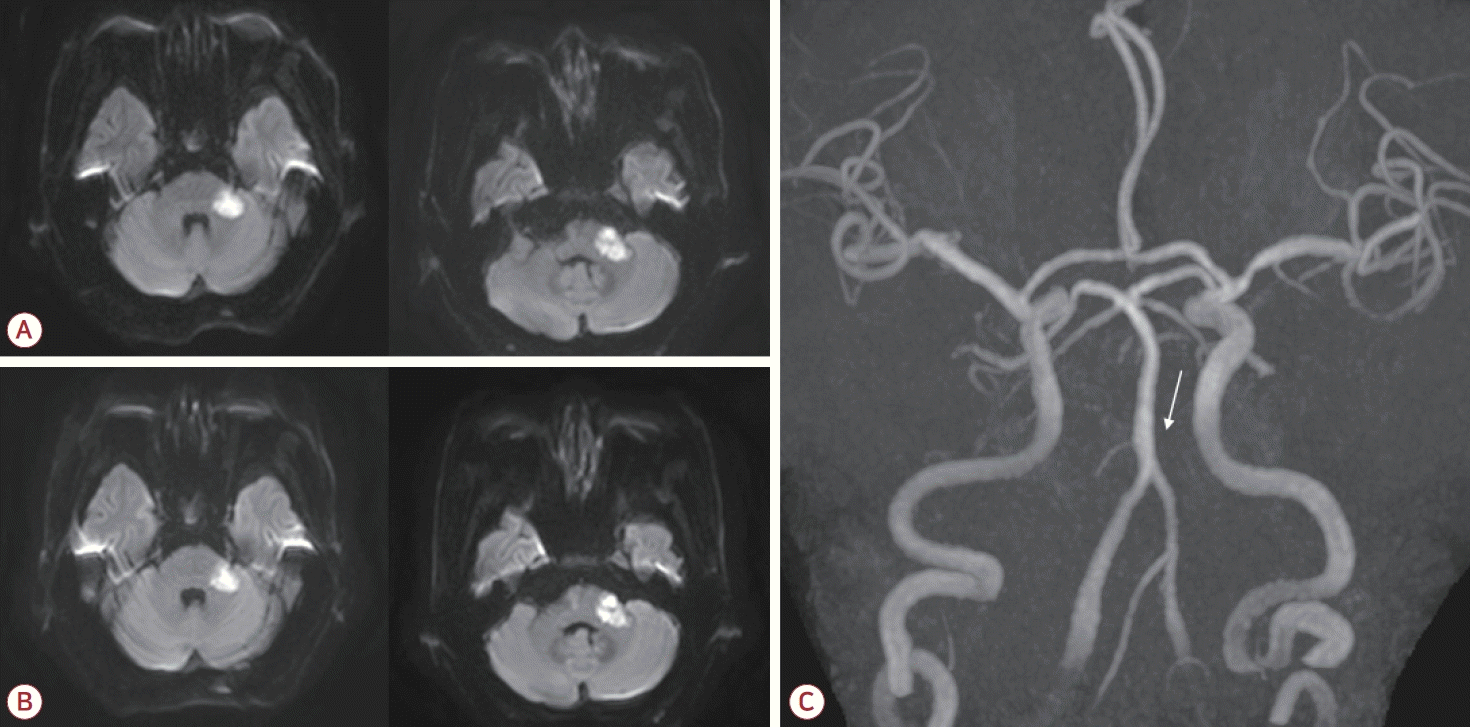
(A) Diffusion-weighted imaging of the brain showed high signal intensities in the left middle cerebellar peduncle and the lateral pontine area. (B) Follow-up imaging performed 6 days later demonstrated maturation of the ischemic lesions, with no significant expansion. (C) Magnetic resonance angiography of the brain indicated nonvisualization of the left AICA where it originated from the basilar artery (white arrow). AICA; anterior inferior cerebellar artery.
High-intensity statin and dual antiplatelet therapy (aspirin and clopidogrel) was initiated for secondary stroke prevention due to the presence of atherosclerotic risk factors. However, at 3 days after the admission (corresponding to 10 days after the initial onset of stroke symptoms) the patient developed new-onset left facial weakness affecting both the upper and lower face. The weakness had a severity of House-Brackmann grade IV, characterized by an inability to wrinkle the left forehead, incomplete closure of the left eye, and mouth asymmetry (Fig. 2). Ocular movements remained intact. A nerve conduction study of the facial nerves was performed. Following stimulation of the left facial nerve, the compound motor action potentials were 31% lower in the left orbicularis oris and orbicularis oculi than on the right side. After stimulating the left supraorbital nerve, the blink reflex test revealed that ipsilateral R1 and R2 responses were absent while the contralateral R2 response was normal, confirming the presence of left facial neuropathy. The brainstem auditory evoked potential did not respond to the left-ear stimulation. Follow-up magnetic resonance imaging (MRI) of the brain was performed to evaluate the new-onset facial palsy, which revealed maturation of the existing ischemic lesions involving the left middle cerebellar peduncle and the lateral pons (Fig. 1-B). Importantly, no new infarction or significant expansion was detected that could explain the delayed facial palsy. The patient was managed conservatively without corticosteroid therapy, and the left facial palsy improved 1 month after its onset.
DISCUSSION
Various combinations of cranial nerve palsies can manifest in AIS depending on the lesion location and vascular supply. For localizing an AIS lesion in the brainstem it would be helpful to categorize the neurological deficit of the cranial nerves into stereotyped classical syndromic norms [4]. The facial motor nucleus is located in the dorsal part of the caudal pons, and the facial nerve fascicles from the facial motor nucleus run around the outside of the abducens nucleus to form the facial colliculus on the floor of the fourth ventricle. They then descend to the ventrolateral side and exit at the pontomedullary junction, and run together with the vestibulocochlear nerve in the internal auditory meatus [5]. The close proximity of the facial motor nucleus to other neural structures typically results in peripheral facial palsy due to brainstem AIS being accompanied by extraocular motor or other cranial nerve dysfunctions. Isolated peripheral facial palsy usually suggests the presence of neuropathy outside the brainstem. However, in rare cases it can manifest as the sole presenting symptom of brainstem AIS or as a delayed, isolated new deficit.
Potential loci for the development of peripheral facial palsy due to AIS in the brainstem include the facial motor nucleus itself, being located in the dorsal part of the caudal pons, the colliculi, acting as the pathway for intra-axial facial nerve fascicles emanating from the nucleus, the lateral part of the pons supplied by the AICA, and the pontomedullary junction or dorsolateral upper medulla through which the intra-axial facial nerve fascicles pass [6-9].
The AICA originates from the BA and provides blood supply to the anterior inferior cerebellum, middle cerebellar peduncle, lateral pons, and inner ear. The main symptoms of AICA infarction are cerebellar dysfunctions and audiovestibular disturbances. The internal auditory artery originating from AICA is an end artery supplying the inner ear and the vestibulocochlear nerve, and its involvement contributes to the audiovestibular symptoms observed in AICA infarction [3]. AICA infarction can also reportedly present with peripheral facial palsy, but the exact location of the lesion has not been clearly defined [2].
Our patient exhibited typical AICA infarction symptoms: ataxia and sensorineural hearing loss. However, peripheral facial palsy developed with a significant delay (10 days after the stroke onset) and, crucially, with no radiological evidence of infarct exacerbation or a new intra-axial lesion on follow-up MRI. This temporal gap and the lack of a new pontine lesion decreased the likelihood of a direct intra-axial mechanism; that is, damage to the facial motor nucleus or nerve fascicles. Although the initial infarct involved the lateral pons, we propose that the delayed palsy resulted from ischemia of the facial nerve along its extra-axial course (at the cerebellopontine angle or internal acoustic meatus), which is a region that is also supplied by AICA branches [3,10]. This suggests the presence of variations in the susceptibility to ischemia, with the pontine tissue infarcting immediately (causing ataxia), and the facial nerve trunk exhibiting delayed dysfunction, and perhaps sustaining only partial ischemia. A similar case of the delayed development of peripheral facial palsy in the AICA infarction has also been reported [10]. In our case, the electrophysiological abnormalities observed immediately after the onset of palsy suggest that ischemic injury preceded the clinical symptoms, consistent with ischemic conduction block. Although pathophysiologically injury may have started earlier, the delayed clinical presentation itself is noteworthy. This highlights that facial palsy can manifest days after the initial stroke, potentially misleading clinicians into suspecting separate etiologies.
We acknowledge that this case report is limited by the absence of high-resolution MRI protocols, such as 3D heavily T2-weighted or contrast-enhanced internal auditory canal sequences, which precluded direct visualization of extra-axial ischemic injury. Nevertheless, an ischemic etiology remains the most plausible since common differential diagnoses such as Bell’s palsy and Ramsay Hunt syndrome were clinically excluded due to the lack of characteristic signs such as retroauricular pain, otalgia, or vesicular eruptions. The distinct temporal association with the AICA infarction further supports this conclusion.
We have encountered a case of delayed peripheral facial palsy (10 days postonset) in a patient with AIS in the territory of the AICA. The presentation suggested that this palsy resulted not from the intra-axial pontine lesion, but from ischemia of the extra-axial facial nerve, which demonstrated a different injury timeline. This case indicates that it is important to not overlook AIS as a possible cause of peripheral facial palsy, even when the onset is delayed or appears isolated, especially when multiple vascular risk factors are present.